Oragenics, Inc. (NYSE: OGEN) is a clinical-stage biotechnology company pioneering the intranasal delivery of pharmaceutical medications for neurological disorders. Its lead candidate, ONP-002, is a first-in-class, novel neurosteroid administered via an innovative intranasal device for the treatment of mild traumatic brain injury (mTBI), commonly known as concussion. With concussions representing a significant market opportunity with no FDA-approved treatments currently available, ONP-002 is uniquely positioned to become the first FDA-approved pharmaceutical therapy for this critical unmet medical need.
TraderTv Interview with Dr. Kelly - 06.24.25
$8.9B
Global Concussion Market Potential Projected to Grow by 2027
3.8M
Approximate Concussions Reported in the US Annually
$40B
Nasal Drug Delivery Platform Market Value Projected to Grow by 2040
Investor Resources:
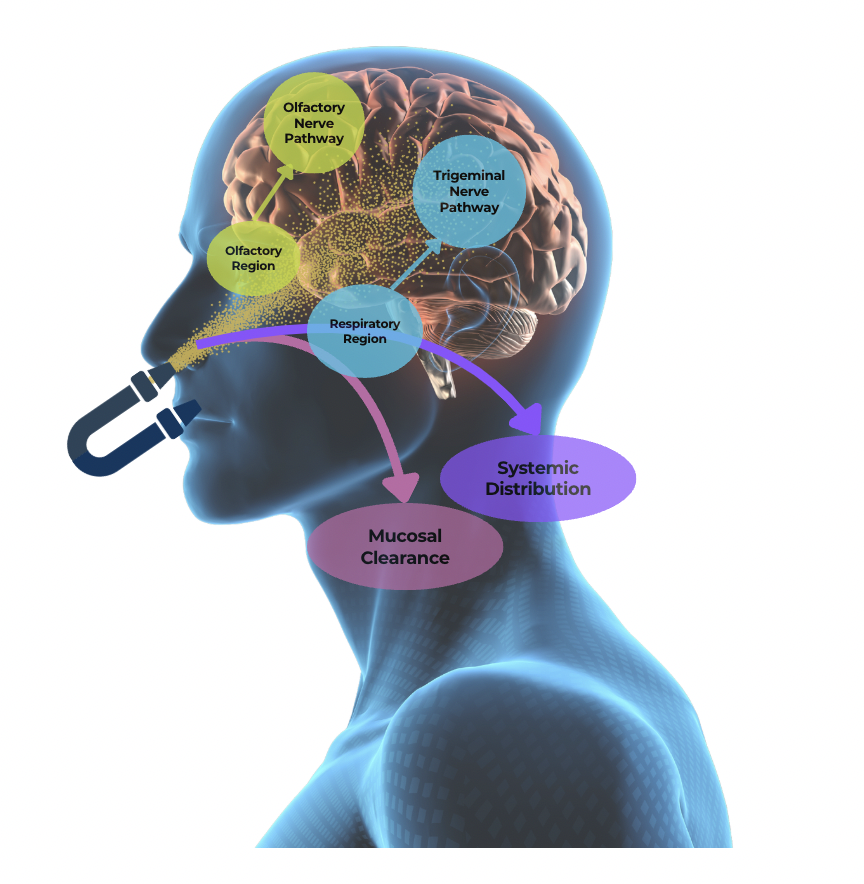
Intranasal Delivery Advantages: Provides rapid and direct access to the brain, enabling swift therapeutic effects. With a quicker onset compared to oral medications, this approach offers an efficient and effective solution for treating neurological conditions like concussions
Innovative Design: Device allows patients to blow into it, elevating the soft palate to enhance drug delivery directly to the brain. This design minimizes systemic exposure, reducing the risk of side effects for a safer and more targeted treatment.
User-Friendly and Portable: Compact and lightweight design ensures ease of use, making it accessible for patients in acute brain injury scenarios.
